Regulatory Setting
It is important that audits are considered in the appropriate regulatory environment and what this is will depend entirely on the scope of the organisation’s activities.
We will not cover them all here, but to follow are a few common standards to illustrate the point.
For example, there are specific laws and regulations within the pharmaceutical, agrochemical and clinical research industries where there are multiple legal requirements, including regulations setting out expected standards for performing certain activities.
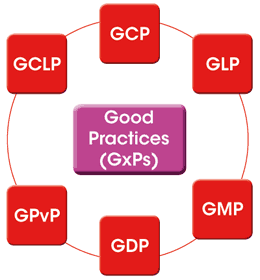
These are collectively known as the Good Practices or GxPs and would include:
- Good Clinical Practice (GCP)
- Good Laboratory Practice (GLP)
- Good Manufacturing Practice (GMP)
Good Manufacturing Practice is also a phrase associated with the food and engineering industries, which also have many of their activities well defined by law.
Additionally legal requirements cover Good Distribution Practice and Good Pharmacovigilance Practice. Good Clinical Laboratory Practice is a standard originally developed by BARQA to meet the needs of clinical laboratories undertaking clinical trials work.
