Fisons
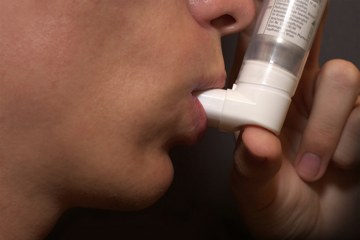
The company Fisons had changed the design of their inhaler products without securing the approval of the Food and Drug Administration (FDA). However, leakage problems were reported with the new inhalers, and a catalogue of failures were discovered at Fisons UK manufacturing plant.
From 1990 to 1995 the company was not receptive to the concerns of the FDA and their Inspectors. This became very confrontational and highlighted the lack of understanding of the cultural differences between the UK and USA legislation.
Competitors moved in with alternative products and Fisons lost market opportunity. Arguably this saw the demise of the company.
