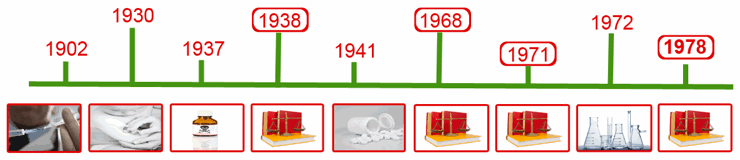
Events Leading to the Introduction of Good Manufacturing Practice
The track record of the early pharmaceutical companies was poor. During several decades a number of high profile cases resulted in significant loss of life and countless injuries. These cases indicate a serious lack of management oversight, lack of manufacturing controls and poor record keeping.
In the 1960s a number of adverse reactions to medicines were being reported which drove the government of the UK, in 1963, to set up the Dunlop Committee whose job it was to oversee adverse events. The most notable of these medicines was thalidomide which was subsequently shown to be teratogenic. This committee later became known as the Committee for the Safety of Medicines (CSM).
The next few pages will explore the high profile cases and subsequent legal outcomes.